Category Archive: Uncategorized
Bronze is an alloy that demonstrates impressive versatility, strength, and aesthetic characteristics as a historically cherished material. From Bronze Age tools to ancient Greek statues, bronze has left its mark on our world. But does bronze corrode and eventually lose its luster? We will explore this question and bronze’s various properties to help you determine if this metal is suitable for your artistic, structural, or industrial project.
Understanding Bronze and Corrosion
Bronze is an alloy composed of primarily copper and tin. Depending on the intended application, the alloy may also contain elements like nickel, zinc, and/or aluminum. Bronze alloy is known for its unique characteristics, including its dark gold appearance, resistance to corrosion, and high strength. These properties make bronze a preferred metal for various artistic and functional applications.
The Bronze Corrosion Process
Corrosion is a natural deterioration process due to several factors, such as environmental conditions like the presence of chemicals, moisture, and extreme temperatures. There are two main types of corrosion: uniform corrosion and localized corrosion. Uniform corrosion spreads across the surface evenly, while localized corrosion affects a specific area of the surface, such as in crevice corrosion, galvanic corrosion, and pitting.
While no material resists corrosion entirely, bronze offers impressive resistance to corrosion due to several factors.
Does Bronze Have Corrosion Resistance?
Bronze offers excellent corrosion resistance through its inherent properties and formation of patina. However, some environments will require extra attention to ensure corrosion resistance. The following properties influence bronze’s corrosion resistance:
- Inherent Resistance: Bronze’s copper content is a primary component of the alloy, forming an oxide layer to protect its surface from oxidation and acting as a corrosion-resistant barrier.
- Protective Patina Formation: A patina is a thin protective layer that develops over the surface of materials like bronze. The patina has a green or blue hue and grants bronze components a natural barrier against deep corrosion. The patina formation depends on the alloy’s specific composition and environmental factors.
- Environmental Factors: While bronze alloys offer exceptional corrosion resistance, certain environmental factors can influence the metal’s ability to resist corrosion formation. Proper maintenance and care are required in environments prone to saltwater, highly polluted atmospheres, extreme humidity, and extreme temperatures to prevent accelerated corrosion.
Does Bronze Corrode With Maintenance and Prevention?
Ensuring the longevity and appearance of bronze components requires routine maintenance and cleaning. Minimizing exposure to moisture and harsh conditions can prevent corrosion, extending the lifespan and appearance of the bronze component. In addition, bronze’s innate corrosion-resistant properties can be enhanced with treatments and protective coatings.
Learn More About Bronze From Sequoia Brass & Copper
While no material is completely impervious to the effects of corrosion, bronze’s innate properties and protective patina offer sufficient corrosion resistance under the right circumstances. When protected from extreme environmental conditions and cared for through routine maintenance, bronze is a long-lasting material in a variety of applications, from sculptures to mission-critical marine components.
Sequoia Brass & Copper is here to help you determine the ideal bronze alloy for your project. We have over 40 years of experience delivering metal fabrication services, and our staff is dedicated to providing the highest quality metal alloys and exceptional customer service. Browse our catalog of bronze products to learn more about our offerings, or request a quote for specific weight, size, and pricing options.
Bronze is an alloy made from copper and tin. Throughout human history, it has been an important material that has greatly contributed to the advancement of civilizations. Today bronze is used in numerous applications, from the automotive industry to architecture, agriculture, and more. One particularly interesting aspect of this metal is its magnetic behavior.
Before deciding to implement bronze in any application, understanding whether or not it is magnetic is an important step. As one of North America’s leading bronze suppliers, Sequoia Brass & Copper can help you with everything you need to know about this valuable metal alloy. In this blog, we’ll explore the magnetic properties of bronze and discuss how its alloying elements influence its overall magnetic behavior.
Bronze Composition
As a copper and tin alloy, bronze is typically composed of around 88% copper and 12% tin. Both of these metals feature unique atomic structures and magnetic properties that influence bronze’s characteristics. In its pure form, copper features no magnetic properties, while tin is lightly attracted to magnetic fields.
Magnetic Properties of Copper
Magnetism is caused by the motion of electrons spinning around an atom’s nucleus. When equal numbers of electrons spin in opposite directions, they are not attracted to a magnetic field. When they spin in the same direction, however, a magnetic field is produced. Copper has a face-centered cubic crystal structure, and features a single valence electron in its outer shell. Due to this unpaired electron, copper exhibits some weak diamagnetic properties.
A diamagnetic material is one that creates a weak magnetic field in opposition to externally applied magnetic fields. While the diamagnetic effect of copper is weak, the metal still demonstrates an inherent aversion to magnetic forces.
Magnetic Properties of Tin
Tin has a body-centered tetragonal crystal structure and, unlike copper, contains two free valence electrons. These electrons cause tin to be weakly paramagnetic. Paramagnetism is caused by a material’s multiple unpaired electrons that are weakly attracted to an externally applied magnetic field.
Similar to copper, tin’s paramagnetic properties are not particularly strong, but still present. When tin and copper are combined into an alloy, their unpaired electrons pair up, creating a non-magnetic material.
Bronze’s Magnetic Behavior
When we have a basic understanding of the metals bronze is made up of, it becomes easier to predict how this material will behave. Bronze’s magnetic behavior is most significantly influenced by the proportion of copper and tin that are used to create it. Since copper makes up the largest proportion of bronze, it contributes its diamagnetic behavior to the alloy.
While tin is a paramagnetic material, it is not dominant enough to affect bronze’s overall magnetism. As a result, bronze’s magnetic behavior is close to diamagnetic, due to its copper dominance. This means that bronze slightly repels a magnetic field, although the effect is significantly weaker than that of pure copper, due to the influence of tin.
The Influence of Impurities in Bronze
While bronze is primarily composed of tin and copper, both historical and modern bronze alloys can contain trace amounts of impurities. Due to variations in ore sources or manufacturing techniques, other metals, non-metals, and metalloids are sometimes found in bronze, such as:
- Phosphorous
- Silicon
- Aluminum
- Manganese
- Nickel
In some cases, these impurities can impact bronze’s magnetic behavior. Impurities that introduce ferrimagnetic or ferromagnetic behavior can make bronze more responsive to magnetic fields. Depending on the type and amount of materials added to bronze, various useful properties can be achieved, such as enhanced machinability or ductility.
When materials like aluminum or manganese are added, bronze becomes weakly magnetic, since both aluminum and manganese are paramagnetic materials. For this reason, it’s important to understand which alloying metals are found in bronze before selecting a certain type for an application.
Learn More About Bronze with Sequoia Brass & Copper
Since bronze is primarily made up of copper, which is diamagnetic, bronze is not magnetic. However, it’s possible for other elements to be added in quantities that subtly affect bronze’s magnetic behavior. With its diverse range of end-use applications, bronze products are a necessity for many industries.
Sequoia Brass & Copper specializes in supplying high-quality alloy bronze metals at competitive prices. All of our bronze products are cut to order according to your specifications. To learn more, visit our bronze page to review our product offerings or request a quote to get started on your next project.
Metalworkers have used bronze for millennia. From the time when civilizations first cast bronze tools around 3000 BCE through today when manufacturers produce high-performance components for vehicles, industrial equipment, and electrical systems, this alloy has offered many advantageous characteristics as compared to other metals..
By combining copper and tin with different materials like aluminum, silicon, and phosphorous, malleable bronze alloys imbue products with long-lasting strength, resistance capabilities, and thermal and electrical conductivity, all with a beautiful surface finish. Learn more about the properties of bronze, how diverse industries use it, and the bronze alloy types that are available.
Bronze Properties
By alloying copper and other metals, bronze takes on the beneficial properties of multiple materials. Some key characteristics that make bronze a popular material choice are:
- Improved tensile strength through alloying
- Resistance to wear, corrosion, and stress
- Ductility and malleability for easy forming and ability to hold shapes
- Thermal and electrical conductivity for heat transfer and electrical projects
- Pleasing aesthetic with attractive coloring, soft shine, and patina development over time
Bronze Applications in the Architectural Industry
Architectural professionals value bronze for its versatility, as its benefits address a structure’s function as well as its form.
Structural Applications
Bronze is both strong and durable enough to use for structural components. This includes building cladding, facades, and domes or roofs. Builders can also restore historical buildings, utilizing bronze to create windows and doors that preserve authenticity, as well as erect plaques or monuments to honor designated locations and people.
Ornamental Applications
Bronze has a warm color and naturally develops a beautiful and protective patina on its surface that resists corrosion. Architects and designers use it for ornamental fixtures and hardware, in addition to more artistic elements like statuary and sculptures.
Bronze Applications in the Industry
Industrial organizations incorporate bronze into their machinery and tools, among other applications.
Manufacturing Equipment Components
Parts within manufacturing equipment often have a bronze composition. Manufacturers use bronze to make couplings, bushings, bearings, gears and wear surfaces.
Tooling & Machining
When manufacturers produce tooling, they’ll utilize bronze for its strength, wear resistance, and formability. It has applications in cutting and shaping tools, dies, and molds.
Marine & Offshore Applications
Because of its corrosion resistance, manufacturers can use bronze to create durable ship parts like shafts and propellers.
Bronze Applications in the Agricultural Industry
The agricultural industry also relies on bronze to create heavy-duty equipment and system components.
Irrigation Systems
Irrigation systems often include bronze fittings, valves, sprinkler parts, pump housings, and more. Bronze can withstand exposure to the elements, water, and pressure.
Farming Equipment Components
In addition to parts for irrigation systems, bronze components are frequently used in harvesting equipment because they offer dependable strength even in such rugged applications.
Bronze Applications in the Automotive Industry
Vehicle designs commonly feature bronze components for internal systems.
Engine Components
Engines rely on precise, heat-resistant metal components for reliable operation. Manufacturers use bronze to produce durable valve guides, bushing, and bearings.
Electrical Systems
Bronze is electrically conductive, making it a good option for connectors, relays, switches, and terminal components in vehicles.
Suspension & Steering
Bronze components in suspension and steering systems include control arms, tie rods, bushings, bearings, and more. Bronze can last for a long time in such applications without incurring wear.
Bronze Alloys
Bronze is primarily composed of copper and tin, but it also includes amounts of aluminum, silicon, phosphorous, and other elements. The properties of a bronze product depend on the composition of the alloy, including the type and proportion of the alloying metals. Some common bronze alloys include:
- Aluminum bronze. This bronze alloy is particularly strong and resists corrosion.
- Silicon bronze. Manufacturers use silicon bronze for its optimal weldability.
- Phosphor bronze. This alloy is electrically conductive and has excellent physical spring characteristics.
Bronze From Sequoia Brass & Copper Inc.
At Sequoia Brass & Copper, we’ve specialized in high-quality nonferrous metals and alloys since 1983. We serve the metal fabrication industry, carrying solid and cored bar bronze shapes as well as bar, sheet, and plate forms of materials like copper and brass.
Our team can cut each of our bronze products to order to best fit your unique needs and close tolerance requirements, offering on-demand custom cutting services in-house. To begin your project, fill out our online form and request a quote today.
Brass is a popular alloy used across all major industries because of its favorable characteristics. Brass is electrically conductive because it contains copper and zinc as its primary elements. Depending on the proportion of copper to zinc, the alloy may be more or less conductive, strong, hard, or machinable. The ratio of elements in the brass can even alter the color of the alloy, making it a popular choice for art, visible home fixtures, and musical instruments.
Learn more about how to evaluate the conductivity of brass, common brass applications, and its conductivity relative to other metals.
Electrical Conductivity Explained
Electrical conductivity is the property that measures how easily electricity travels through a material. Resistant materials with low conductivity do not transfer electricity efficiently; those materials can be used as insulation. Very conductive materials allow electricity to pass through them quickly with very little electrical loss, making them an excellent choice for electrical components like wires and relays.
The degree to which brass and other alloys are conductive depends on the exact composition of the metal. If it has a higher proportion of conductive metal (like copper) and a lower proportion of resistant material (like zinc), the overall conductivity of the alloy will be high. In industries like telecommunications, electrical engineering, and electronics, it’s important to use metals that are properly conductive so they can efficiently send electrical signals with minimal loss or heat generation.
Evaluating the Conductivity of Brass
To have the right type of brass for every application, manufacturers need to source reliably fabricated alloys with known compositions. Manufacturers can also use a variety of factors to evaluate the stock’s conductivity. Some of the conditions that affect the conductivity of individual pieces of brass include:
- The proportions of different metals within the alloy
- Ambient temperature
- Surface condition
Brass Conductivity: Experimental Evidence
While manufacturers can rely on product records to determine the conductivity of their brass supply, they can also conduct tests and experiments to calculate the precise level of conductivity. Scientists have run significant tests on different brass alloys to create records and mechanisms for ascertaining their properties.
Individuals can also test the conductivity of brass by putting it to use. For example, they can test electrical wires, connectors, and other components to measure their effectiveness. Musical instruments can also be tested by the instrument’s tonality, which is also affected by the metal’s conductivity.
Applications of Brass
The inherent conductivity of brass makes it an excellent material option for myriad applications. Some of the most common applications for brass include:
- Decorative elements: A popular choice for complex architectural constructions. Its appealing color and formability have also historically made it a popular choice for complex sculpture art.
- Electrical and electronics: Brass offers high levels of conductivity and low levels of resistance, which is ideal for electrical wires, connectors, switches, and other internal components.
- Musical instruments: The conductive properties of brass improve the tonal quality of brass instruments, such as trombones, trumpets, and saxophones.
- Plumbing: Plumbing systems use brass components for controlling the movement of liquid and gas throughout pipes with minimal static electricity.
Factors Influencing Material Selection
Brass offers several beneficial characteristics to manufacturers, including easy fabrication, resistance to corrosion, and electrical conductivity. However, its conductivity is lower than copper, aluminum, silver, and gold, so brass is ideal for applications that need some electrical conductivity and don’t require investing in more expensive copper materials.
Brass From Sequoia Brass & Copper
At Sequoia Brass & Copper, we provide our clients with high-quality brass stock that is cut to order and quick to ship. Select products from our brass inventory, or request a quote to start your order.
What Is Phosphor Bronze?
Phosphor bronze is a copper alloy that is commonly used for applications requiring a high degree of corrosion resistance and electrical conductivity. The composition of this alloy includes a mixture of copper tin and phosphorus. Other metals such as lead are also commonly included to achieve certain desirable properties.
Benefits and Properties of Phosphorus Bronze
Due to its combination of copper, tin, and phosphorus, this material offers a wide range of desirable attributes. This highly valuable, specialty metal offers excellent formability, solderability, fatigue resistance, and spring qualities that make it a popular choice for several industries and applications.
Other key benefits and properties of phosphor bronze include the following:
- High corrosion resistance. Phosphor bronze resists corrosion and rusting in the face of physical elements (such as humidity and rain) or chemical exposure.
- Electrical conductivity. The metal can conduct electricity well, making it a popular option for manufacturing electrical parts.
- Strength. The inclusion of tin and phosphorus gives this metal added strength, as well as the ability to resist fatigue and wear.
- Elasticity. Due to phosphor bronze’s fine grain size, the material is highly elastic and offers a greater degree of springback.
Industry Applications
Because of its favorable characteristics, manufacturers across a wide array of industries use it to produce a variety of specialty components. Any industry that requires high-density, high-performance components that resist damage from corrosion, fatigue, and mechanical wear can turn to phosphorus bronze for a versatile, easily formable option. Whether used in wire, strip, or rod form, phosphor bronze can be found in various applications, such as:
Automotive
Due to its electrical conductivity and other beneficial properties, phosphor bronze is used to produce a variety of automotive electrical parts, including terminals, lighting components, cable harnesses, connectors, springs, and relays.
Electronics
Manufacturers across the electronics industry use phosphor bronze to produce a variety of electronic components, including:
- Circuit breaker contacts
- Connectors
- Fuse clips
- Relay contacts
- Rotary switch slides
- Transistor terminals
Industrial
Phosphor bronze offers great fatigue, wear, and corrosion resistance, making it an ideal material for a variety of industrial applications. The most common industrial uses include fasteners, bolts, springs, truss wire, diaphragms, bushings, and other machinery components.
Musical Instruments
Compared to brass, another popular metal in the musical industry, phosphor bronze offers a higher level of resiliency. When used to produce musical instruments, phosphor bronze can deliver a larger tonal spectrum and more consistent sounds over time. This metal is commonly used in trombones, trumpets, and reed instruments such as saxophones.
Alloy Metals by Sequoia Brass & Copper
Choosing the right metal alloy is essential in ensuring the success of your project. While phosphor bronze may be an ideal option for the above use cases, other metals may be the stronger choice for different industries and applications. At Sequoia Brass & Copper, we offer a variety of copper, brass, and bronze alloys in bar, plate, and sheet forms. We’re here to assist you. Please contact us or request a quote for more information.
Rusting and corrosion are undesirable effects that can ruin a metal’s ability to perform optimally for the intended application. When working with environments exposed to moisture, chemicals, and other corrosive substances, it’s important to select metal materials that are resistant to corrosion and rust.
At Sequoia Brass and Copper, we are a domestic supplier with decades of experience serving the metal fabrication industries in North America. We are a service-oriented company, committed to offering the highest quality materials and customer service. As part of our product catalog, we offer a range of corrosion- and rust-resistance materials, including copper, brass, and bronze.
What Causes Metals to Corrode?
Metal corrosion is a natural process that gradually deteriorates metal materials via an electrochemical reaction. This reaction begins on the metal’s surface and involves the exchange of electrons between the metal and the corroding agent, such as oxygen or water. Corrosion of metals can occur due to exposure to environmental elements such as humidity and condensation. The metal reacts with the hydrogen and oxygen in the water, causing it to oxidize.
What Causes Metals to Rust?
Rust is the result of corrosion, which is caused by metal oxidation. During oxidation, the metal wears away and develops a visible red or brownish substance (rust) on the surface. If a metal susceptible to corrosion is uncoated, its lifespan is significantly shorter than one with the proper protective coating.
Rust must be treated; otherwise, it can completely corrode a metal into a dry oxide powder. The most common environmental elements that lead to rust include chemicals, salt, moisture, and air.
Metals That Are Corrosion Resistant
At Sequoia Brass and Copper, we offer the following types of corrosion-resistant metals:
Copper
Copper is excellent in applications requiring the conduction of heat and electricity. The material exhibits relative corrosion resistance properties, making it ideal for various metal fabrication applications. Sequoia Brass and Copper specializes in Oxygen-Free copper, and we maintain a significant inventory of all copper products at our warehouse in Hayward, California. We carry copper in various stock sizes, ensuring you get the material you need with competitive prices and shipping rates.
Brass
Composed of copper and zinc, brass is commonly used for electric components, musical instruments, automotive parts, architectural structures, and more. This corrosion-resistant metal features a low melting point and can be easily cast. To meet a range of needs, we carry brass in various stock sizes and forms, including tube, plate, rod, sheet, bar, and pipe.
Bronze
Bronze is created by alloying copper and tin in varying amounts. This metal is harder than brass and provides durable, sturdy, and corrosion-resistant performance. These desirable properties make it a common choice in industries such as automotive, industrial, architectural, and agricultural. Additionally, it is non-sparking and produces minimal friction, making it suitable for producing weapons, tools, and bells. At Sequoia Brass and Copper, we can provide bronze materials in cored bar and solid bar forms, with various stock sizes available.
Other Corrosion Resistant Metal Examples
In addition to the materials listed above, other examples of corrosion-resistant metals include:
Stainless Steel
Stainless steel is a mixture of elements, and most have some iron traces, making it oxidize quickly to form rust. However, many stainless steel alloys have chromium which oxidizes to form chromium oxide on the metal surface. This protective layer is corrosion-resistant and hinders oxygen from reaching the underlying steel. Stainless steel also has other elements, such as molybdenum and nickel, which increases its rust resistance.
Aluminum Alloy
Aluminum is ideal for manufacturing automotive and aircraft parts due to its lightweight and corrosion-resistance properties. The material doesn’t contain iron, meaning it won’t rust. Additionally, it oxidizes when exposed to water, which causes an aluminum oxide film to form on the surface. This film prevents further corrosion and protects the metal underneath.
Galvanized Steel
Although galvanized steel will eventually rust, the process takes a relatively long time. Galvanized steel is a type of carbon steel that has undergone galvanizing or has been coated with zinc. The zinc coating hinders oxygen and water from reaching the underlying steel, thus offering advanced corrosion protection.
Source Your Corrosion-Resistant Materials from Sequoia Brass and Copper
To ensure you get the right corrosion-resistant materials for your metal fabrication needs, partner with Sequoia Brass and Copper. We have a large inventory of copper, brass, and bronze metals that are available in various shapes and sizes to meet your particular requirement. With a dedication to quality materials and customer service, we can provide you with the metal products you need at competitive prices.
For more information about our corrosion-resistant metals, or to get started on your order, request a quote today. You can also reach our team at (510) 887-5525.
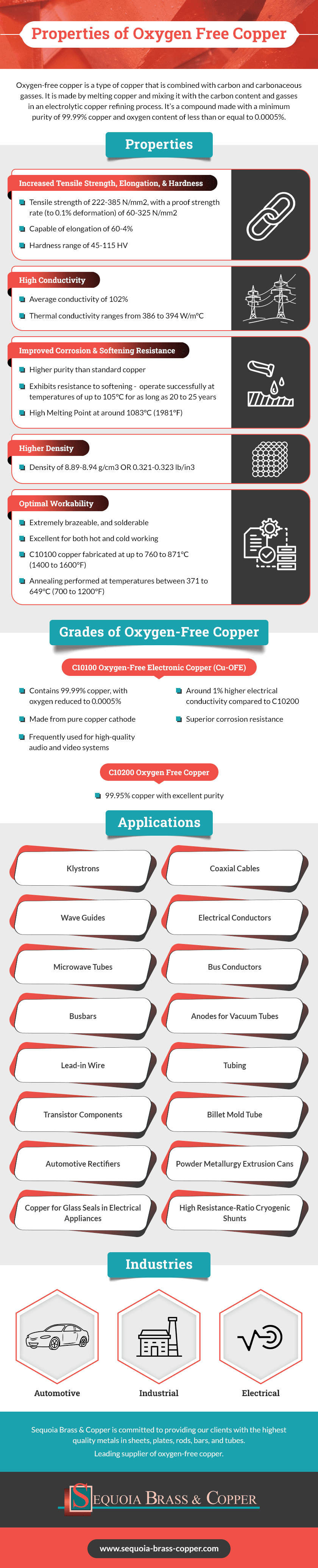
Oxygen-free copper is a type of copper that is combined with carbon and carbonaceous gasses. It is made by melting copper and mixing it with the carbon content and gasses in an electrolytic copper refining process. Subsequently, most of the oxygen contained in the copper is removed, ultimately forming a compound made with a minimum purity of 99.99% copper and oxygen content of less than or equal to 0.0005%.
This pure copper content makes it more durable, thermally and electrically conductive. These and other enhanced properties make oxygen-free copper a popular choice for many applications across a wide range of industries.
Properties of Oxygen-Free Copper
The properties of oxygen-free copper include:
Increased Tensile Strength, Elongation, and Hardness
One of the key properties that make this material popular is its improved tensile strength of 222-385 N/mm2, with a proof strength rate (to 0.1% deformation) of 60-325 N/mm2. This material is also capable of elongation of 60-4% and features a hardness range of 45-115 HV.
High Conductivity
When it comes to conductivity, oxygen-free copper features an average conductivity of 102% along with a guaranteed value of 101% as per IACS. Thermal conductivity also ranges from 386 to 394 W/m°C.
Improved Corrosion and Softening Resistance
Unlike standard copper, oxygen-free copper’s higher purity provides increased corrosion resistance. It also exhibits resistance to softening, meaning it can operate successfully at temperatures of up to 105°C for as long as 20 to 25 years. In addition, it has a high melting point of around 1083°C (1981°F).
Higher Density
If you require metal with greater density, oxygen-free copper is also beneficial in this area. This metal boasts a density of 8.89-8.94 g/cm3 or 0.321-0.323 lb/in3.
Optimal Workability
Oxygen-free copper is extremely brazeable, and solderable. C10100 copper is fabricated at temperatures as high as 760 to 871°C (1400 to 1600°F), and it is excellent for both hot and cold working. Annealing of this grade is also performed at temperatures between 371 to 649°C (700 to 1200°F).
Other properties of oxygen-free copper include low volatility under high vacuum, invulnerability to hydrogen embrittlement, and high impact strength.
Grades of Oxygen-Free Copper
For different applications, there are two main grades of oxygen-free copper available, including:
C10100 Oxygen-Free Electronic Copper (Cu-OFE)
With silver removed, C10100 oxygen-free electronic copper contains 99.99% copper, with oxygen reduced to 0.0005%. This grade is made from pure copper cathode, which is poured beneath a protective gas atmosphere. It boasts around 1% higher electrical conductivity compared to C10200 oxygen-free copper, along with superior corrosion resistance. It’s frequently used for high-quality audio and video systems.
C10200 Oxygen-Free Copper (Cu-OF)
This grade contains 99.95% copper and boasts excellent purity. It’s worth noting that no copper is ever 100%.
Applications/Industries Served by Oxygen-Free Copper
Some common industries and applications for oxygen-free copper include, but are not limited to:
- Automotive, industrial, and electrical industries
- Klystrons
- Coaxial Cables
- Wave Guides
- Electrical Conductors
- Microwave Tubes
- Bus Conductors
- Busbars
- Anodes for Vacuum Tubes
- Lead-in Wire
- Tubing
- Transistor Components
- Billet Mold Tube
- Automotive Rectifiers
- Copper for Glass Seals in Electrical Appliances
- High Resistance-Ratio Cryogenic Shunts
- Powder Metallurgy Extrusion Cans
Contact Sequoia Brass & Copper Today
If you’re looking for superior copper materials that exhibit increased strength, durability, workability, and other benefits, consider using oxygen-free copper from Sequoia Brass & Copper. We are a leading supplier of oxygen-free copper as well as other top-grade nonferrous metals. Our experienced professionals will work with you throughout the metal procurement process to make sure you get only the products you need. We regularly monitor, review, and upgrade our Quality Management Systems to maintain consistent quality, ensure the satisfaction of our customers, and meet all statutory and regulatory requirements.
For more information about our oxygen-free copper materials, request a quote or contact us today.
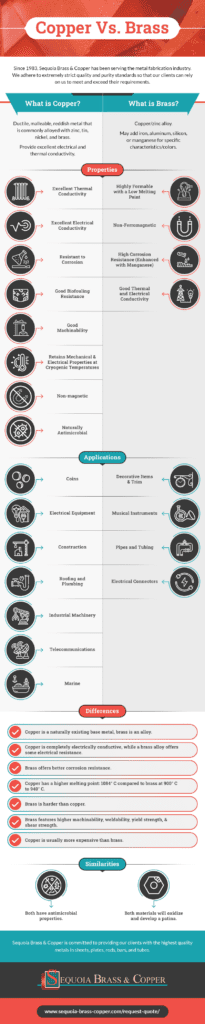
When choosing a material for your project, it’s important to select one with the appropriate qualities to best support your application. Understanding the difference between brass and copper is essential when making these decisions. At Sequoia Brass & Copper, we specialize in providing high-quality materials, including oxygen-free copper, brass, and bronze, for metal fabricators. If you’re considering brass vs. copper, we can help you determine which one is right for you.
What Is Copper?
Copper is a naturally occurring metal that’s highly malleable, though it’s not very hard nor strong. It’s non-magnetic and resists corrosion, with good thermal, conductivity, excellent electrical conductivity and low chemical reactivity. Because it retains its electrical and mechanical properties even at cryogenic temperatures, copper finds a number of uses across industries:
- Coins
- Electrical equipment
- Construction and roofing
- Automotive
- Pipes and plumbing
- Industrial machinery
- Telecommunications
- Marine applications
What Is Brass?
Brass is a copper/zinc alloy, though other metals (like iron, aluminum, silicon, or manganese) may be added to enhance specific characteristics and colors. A higher level of zinc or silicon will make the brass stronger, for example, while the addition of iron will give it magnetic qualities.
Brass is highly formable with a low melting point, and it’s non-ferromagnetic, making it easy to separate to recycle. Desirable brass properties include high corrosion resistance, which can be improved even further by including a larger amount of manganese in the brass, and good thermal and electrical conductivity. However, it is likely to crack if placed under significant stress.
Brass is commonly used in a variety of applications:
- Decorative items and trim
- Musical instruments
- Pipes and tubing
- Electrical components
What Is the Difference Between Copper and Brass?
While copper is a naturally existing base metal, brass is an alloy: a man-made combination of copper and zinc. Brass can feature a variety of characteristics depending upon how much zinc and other metals are mixed with the copper, but brass and copper properties are similar. Copper is antimicrobial, which makes it useful in high-touch applications and medical facilities. Because brass contains copper, it retains those antimicrobial properties. Both materials will oxidize and develop a patina, which for some applications is a desired decorative look. If not, you can seal the brass or copper item to prevent exposure to air.
With its high conductivity, copper is a popular choice for wires and electrical components, while various types of brass find more uses in industrial and consumer goods. Take a look at these key differences between the materials to help you determine whether brass or copper is a better choice for your application:
- Copper is completely electrically conductive, while a brass alloy offers some electrical resistance, depending on the amount of each alloyed metal.
- Brass offers better corrosion resistance.
- Copper has a higher melting point: 1084° C compared to brass at 900° C to 940° C.
- Brass is harder than copper, and the more zinc the brass alloy contains, the harder it will be.
- Brass features higher machinability, weldability, yield strength, and shear strength.
- Copper is usually more expensive than brass, though it can depend on the specific alloy and the material quality.
Whether you go with brass or copper will depend on the environmental conditions and unique stresses your finished product will face. Each metal offers advantages for specific applications.
At Sequoia Brass & Copper, we source copper, bronze, and brass alloys in sheets, tubes, bars, plates, and rods, and we specialize in hard-to-find shapes and alloys. Since 1983, we’ve been committed to working with only high-quality metals while offering competitive pricing, clear communication, quick service, and expert guidance for our clients. With our years of experience in the industry with both domestic and international metal sources, we can connect you with the materials you need for projects of all sizes. Contact us to learn more, or request a quote for your project.
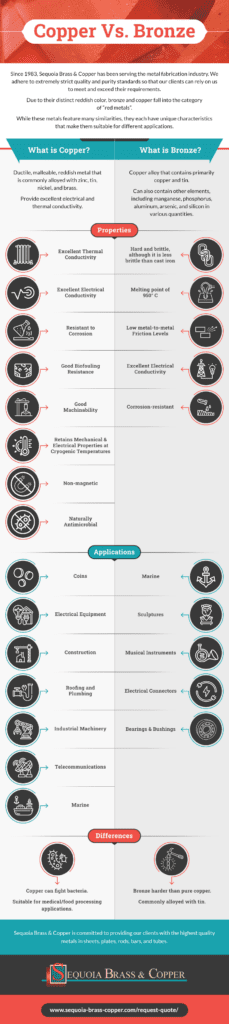
Since 1983, Sequoia Brass & Copper has been serving the metal fabrication industry with high-quality bronze, copper, brass, and other nonferrous metals in various shapes and forms. We adhere to extremely strict quality and purity standards so that our clients can rely on us to meet and exceed their requirements.
Due to their distinct reddish color, bronze and copper fall into the category of “red metals”. While bronze is a copper-based alloy, bronze exhibits more hardness than pure copper and typically contains other elements that produce various properties. While these metals feature many similarities, they each have unique characteristics that make them suitable for different applications.
What Is Copper?
Copper is a ductile, malleable, reddish metal that is commonly alloyed with zinc, tin, nickel, and brass to yield different properties. Copper and copper alloys provide excellent electrical and thermal conductivity, which makes them popular for use in electronics and a wide range of other uses. It can also be easily soldered, or brazed.
Copper Applications
Copper’s many desirable features make it suitable for a wide range of applications, including:
- Coins
- Electrical equipment
- Construction
- Roofing and plumbing
- Industrial machinery
- Telecommunications
- Marine
Copper Properties
Properties of copper include:
- Excellent thermal conductivity
- Excellent electrical conductivity
- Resistant to corrosion
- Good biofouling resistance
- Good machinability
- Retains mechanical and electrical properties at cryogenic temperatures
- Non-magnetic
- Naturally antimicrobial
What is Bronze?
Bronze is copper alloy that contains primarily copper and tin. Bronze can also contain other elements, including manganese, phosphorus, aluminum, arsenic, and silicon in various quantities to produce different properties.
Applications
Bronze alloys are preferred in a wide variety of applications, including:
- Marine. Due to its corrosion resistance, bronze is a popular choice for boats and other marine-exposed applications such as ship propellers and fittings.
- Sculptures. Its muted gold tone and aesthetic appearance make bronze a common material for sculptures and other pieces of art.
- Musical instruments. Bells, cymbals, and other musical instruments are often made of bronze.
- Electrical connectors. Certain varieties of bronze alloys provide great electrical conductivity, making them useful in a wide range of electrical applications such as connectors and springs.
- Bearings and bushings. Bronze alloys are good for bushings and bearings in high-stress environments because of their minimal metal-to-metal friction levels.
Bronze Properties
Bronze alloys are reddish-brown in color and possess properties that enable them to be used in many applications, such as those listed above. The properties of bronze include:
- Hard and brittle, although it is less brittle than cast iron
- Melting point of 950° C
- Low metal-to-metal friction levels
- Excellent electrical conductivity
- Corrosion-resistant
What are the Differences Between Copper and Bronze?
There are many differences between bronze and copper. Bronze is an alloy, or mixed metal, consisting primarily of copper as well as various other elements. Because bronze is commonly alloyed with metals like tin, it is much harder than pure copper. While bronze is easier to fuse and cast, copper has an impressive ability to fight bacteria, making it suitable for industries such as medical and food processing.
Partner With Sequoia Brass & Copper
Both bronze and copper have an impressive range of properties that make them suitable for use in various applications and industries. Sequoia Brass & Copper is committed to providing our clients with the highest quality metals in sheets, plates, rods, bars, and tubes. To learn more about bronze, copper, or other metals we provide, contact us or request a quote today.
Beryllium copper—also referred to as spring copper, BeCu, copper beryllium, and beryllium bronze—is a copper-based alloy that contains varying amounts of beryllium. The beryllium content typically ranges between 0.4 to 2%.
As one of the highest strength copper-based alloys, this material finds application across all sectors of industry. In addition to its high strength, different grades of beryllium copper also demonstrate other properties suitable for various industrial applications, such as excellent conductivity and non-magneticity.
Properties of Beryllium Copper
Beryllium copper and its range of alloys exhibit a wide range of properties, including:
- High electrical and thermal conductivity
- Non-sparking and non-magnetic characteristics
- High ductility and excellent formability with regards to forming, machining, and metalworking processes
- Higher resistance to corrosion and oxidation than steel
- Higher durability than most copper alloys (resists wear and galling)
- Retention of properties in extremely low or extremely high temperatures
- Suitability for precipitation-hardening operations
- High fatigue strength—also known as endurance strength—making it suitable for heavy cyclic load applications
Grades of Beryllium Copper
Beryllium copper is available in a number of different grades, each with its own unique advantages. These grades are classified into two main categories: high strength alloys and high conductivity alloys.
Examples of high strength alloys include:
- This alloy is the most common grade of beryllium copper. It contains 1.8 to 2% beryllium and demonstrates tensile strength ranging between 60–220 kilopounds per square inch (ksi) and fatigue strength between 30–50 ksi per 10^8 cycles.
- This alloy also contains 1.8 to 2% beryllium. It exhibits good machinability, high endurance, relaxation resistance, and a characteristic strength-conductivity ratio.
- This alloy contains 1.6 to 1.79% beryllium. Its electrical conductivity percentage typically falls between 15 to 33% (per International Annealed Copper Standard).
Some of the high conductivity alloys include:
- This alloy contains 0.2 to 0.6% beryllium. It demonstrates an electrical conductivity percentage of at least 60%.
- This alloy contains 0.4 to 0.7% beryllium. Its tensile strength generally falls between 50–80 ksi.
- This alloy contains some of the lowest levels of beryllium among the various grades available, falling within a range of 0.15 to 0.5%. It offers a low elongation percentage (7 to 17%).
- This alloy was created by Brush Wellman. It contains between 0.15 to 0.5% beryllium, is supplied hardened, and demonstrates high mechanical strength and electrical conductivity.
Applications/Industries Served by Beryllium Copper
Beryllium copper finds use in several different industries due to its unique properties. For instance, its non-sparking characteristics make it highly suitable for applications within the oil and gas industry, such as for oil rig components or mining tools.
Other industrial applications which employ beryllium copper include:
- Computers and electronics
- Fabrication of non-sparking tools
- Fasteners
- Telecommunication products
- Valve seats and cryogenics
- Injection mold design
- Musical instruments (percussion, strings, etc.)
Contact Sequoia Brass & Copper Today
Available in several grades with different characteristics, beryllium copper’s versatility is hard to match. At Sequoia Brass & Copper, we offer an extensive selection of copper products, including beryllium copper. Our inventory includes oxygen-free electronic (OFE)/oxygen-free high thermal conductivity (OFHC) copper plates, rods, sheets, and tubes, deoxidized high phosphorus (DHP) copper tubes, and tellurium-copper rectangular bars and rods.
If you’d like to learn more about our material offerings, contact us, or request a free quote today.