Along with copper, bronze and brass belong to a category of metals referred to as “red metals” due to their distinct reddish color. These two materials are copper-based alloys containing varying amounts of other elements that produce a wide range of different properties.
For instance, bronze typically consists of copper and tin, but other elements may also feature in the composition. Regardless of the elemental addition, bronze demonstrates greater hardness than pure copper. On the other hand, brass mainly contains copper and zinc, the latter of which allows for enhanced strength and ductility.
Although there are similarities between brass and bronze, the following post focuses on the individual characteristics, properties, and benefits of each material and the differences between them.
What Is Brass?
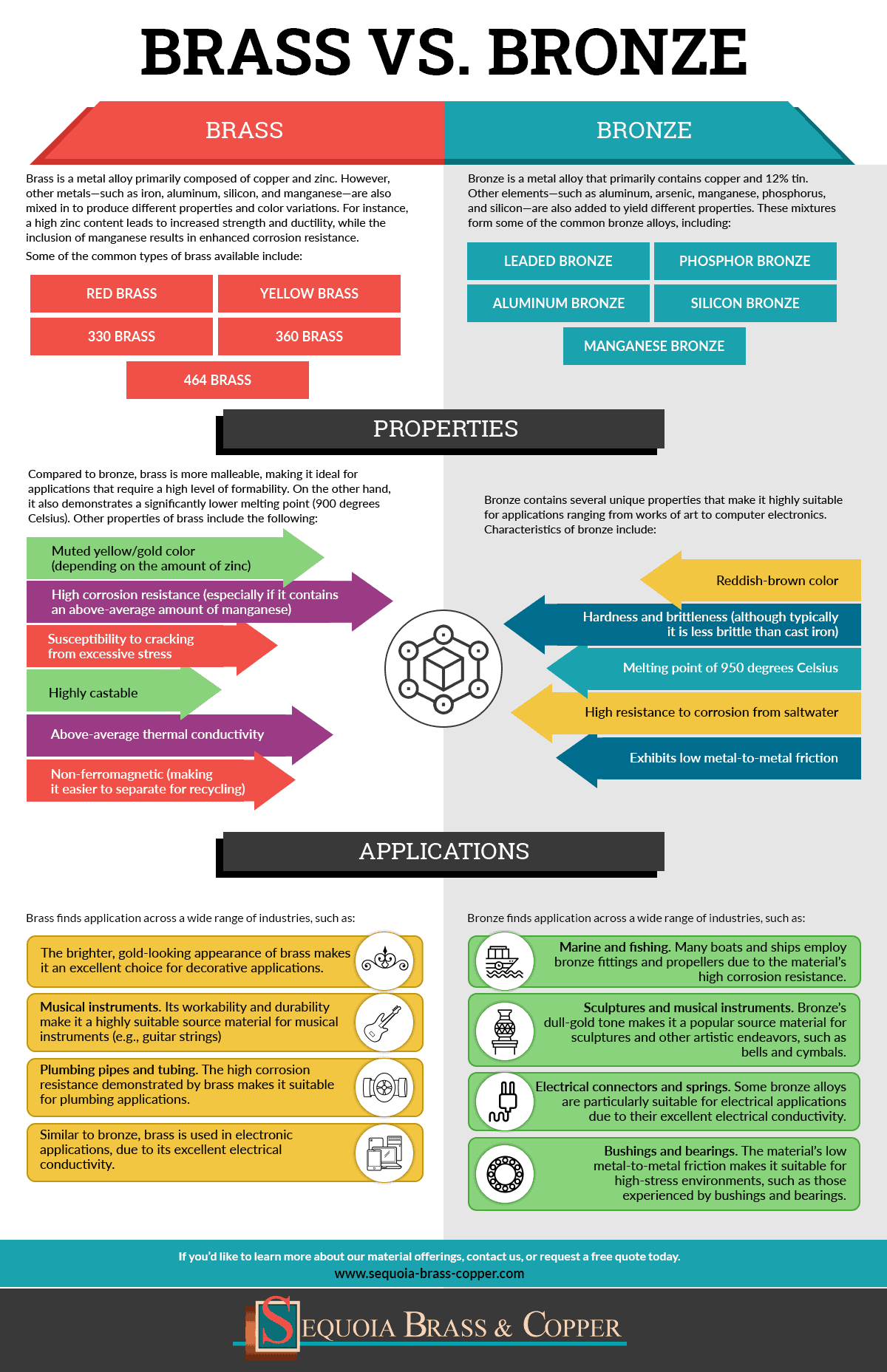
Brass is a metal alloy primarily composed of copper and zinc. However, other metals—such as iron, aluminum, silicon, and manganese—are also mixed in to produce different properties and color variations. For instance, a high zinc content leads to increased strength and ductility, while the inclusion of manganese results in enhanced corrosion resistance.
Some of the common types of brass available include:
- Red brass
- Yellow brass
- 330 brass
- 360 brass
- 464 brass
Properties of Brass
Compared to bronze, brass is more malleable, making it ideal for applications that require a high level of formability. On the other hand, it also demonstrates a significantly lower melting point (900 degrees Celsius).
Other properties of brass include the following:
- Muted yellow/gold color (depending on the amount of zinc)
- High corrosion resistance (especially if it contains an above-average amount of manganese)
- Susceptibility to cracking from excessive stress
- Highly castable
- Above-average thermal conductivity
- Non-ferromagnetic (making it easier to separate for recycling)
Applications of Brass
Brass finds application across a wide range of industries, such as:
- The brighter, gold-looking appearance of brass makes it an excellent choice for decorative applications.
- Musical instruments. Its workability and durability make it a highly suitable source material for musical instruments (e.g., guitar strings)
- Plumbing pipes and tubing. The high corrosion resistance demonstrated by brass makes it suitable for plumbing applications.
- Similar to bronze, brass is used in electronic applications, due to its excellent electrical conductivity.
What Is Bronze?
Bronze is a metal alloy that primarily contains copper and 12% tin. Other elements—such as aluminum, arsenic, manganese, phosphorus, and silicon—are also added to yield different properties. These mixtures form some of the common bronze alloys, including:
- Leaded bronze
- Phosphor bronze
- Aluminum bronze
- Silicon bronze
- Manganese bronze
Properties of Bronze
Bronze contains several unique properties that make it highly suitable for applications ranging from works of art to computer electronics. Characteristics of bronze include:
- Reddish-brown color
- Hardness and brittleness (although typically it is less brittle than cast iron)
- Melting point of 950 degrees Celsius
- High resistance to corrosion from saltwater
- Exhibits low metal-to-metal friction
Applications of Bronze
Bronze’s characteristics make it suitable for use in functional and aesthetic applications, such as:
- Marine and fishing. Many boats and ships employ bronze fittings and propellers due to the material’s high corrosion resistance.
- Sculptures and musical instruments. Bronze’s dull-gold tone makes it a popular source material for sculptures and other artistic endeavors, such as bells and cymbals.
- Electrical connectors and springs. Some bronze alloys are particularly suitable for electrical applications due to their excellent electrical conductivity.
- Bushings and bearings. The material’s low metal-to-metal friction makes it suitable for high-stress environments, such as those experienced by bushings and bearings.
The Differences Between Bronze and Brass
The differences in material compositions between bronze and brass result in varying characteristics that make them suitable for different use cases. For instance, bronze’s higher level of resistance to saltwater corrosion makes it a better choice for ship components than brass, while brass’s exceptional workability and machinability make it more suitable for tubing and pole applications. Table 1 below outlines some of the major differences between the two materials.
Table 1 – Differences Between Bronze and Brass
| Bronze | Brass |
| Harder, more brittle | Greater malleability |
| Melting point of 950 degrees Celsius | Melting point of 900 degrees Celsius |
| Excellent corrosion resistance (incl. saltwater) | Good corrosion resistance |
| Suitable for some decorative applications (e.g., sculptures, musical instruments, etc.) |
More suited for decorative applications (due to gold color) |
| Dates back to 3500 BCE | Dates back to 500 BCE |
Contact Sequoia Brass & Copper Today
Brass and bronze are two copper-based alloys that offer a variety of characteristics suitable for a wide range of applications. At Sequoia Brass & Copper, we offer an extensive selection of bronze and brass materials in bar, plate, tube, rod and sheet form to suit your unique application. If you’d like to learn more about our material offerings, contact us, or request a free quote today.